Some batches of Coryx Throat Spray recalled due to faulty mechanism
Cipla Medpro, in collaboration with the Namibian Medicines Regulatory Council (NMRC), has recalled some batches of Coryx Throat Spray (registration number 13/16.3/0219, scheduling status NS1).
The reason for the Class I Type A product recall is the remote possibility that the spray nozzle may detach from the spray mechanism during use, a statement released on Tuesday said.
“The safety and well-being of our patients is our utmost priority. Therefore, after liaising with NMRC, it was deemed necessary to recall specific batches of this product from the market. We are working closely with spray mechanism manufacturers to ensure there is no margin for error in the future,” said Cipla South Africa Chief Executive, Paul Miller.
According to Miller, people who purchased any of these batches of Coryx Throat Spray are requested to return it to their pharmacy, and they will be refunded the purchase price.
Coryx Throat Spray is indicated for the temporary relief of pain and discomfort in sore throats due to colds (and accompanying tickling cough). It is also recommended for minor mouth irritations. The product is packed in an amber glass bottle with a screw cap closure. The spray mechanism is co-packed separately in the carton.
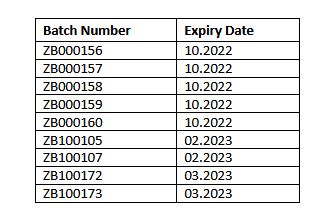
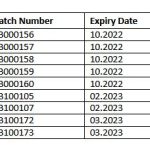 The following batches of Coryx Throat Spray (registration number 13/16.3/0219, scheduling status NS1 have since been recalled.
The following batches of Coryx Throat Spray (registration number 13/16.3/0219, scheduling status NS1 have since been recalled.