Administering of the Pfizer/BioNTech COVID-19 vaccine to children and adolescents commences
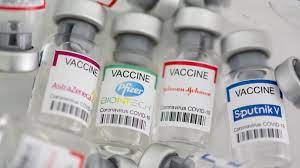
The Ministry of Health and Social Services has decided to expand its vaccination eligibility to children and adolescents aged 12 to17 years, following the World Health Organization’s Strategic Advisory Group of Experts (SAGE) recommendation that the Pfizer/BioNTech COVID-19 vaccine is suitable for use by persons aged 12 years.
The same vaccine has been granted Emergency Use Authorization by the US Food and Drug Administration (FDA) for use in children and adolescents aged 5 to 15 years and full approval for use in people aged 16 years and older, the ministry said in a statement.
The Health Ministry executive director, Ben Nangombe in a statement on Monday said it has commenced with the administration of the Pfizer/BioNTech COVID-19 vaccine to the above age group.
“The vaccine is administered in two doses. Each dose is 0.3 ml. The second dose is given 21 days after the first dose. The vaccine is available and is being offered at all vaccination sites across the country,” he added.
Nangombe in the notification said, informed consent for vaccination of children aged 12 to13 years shall be obtained from parents or guardians who opt to have their children vaccinated, while adolescents aged 14 to 17 years, will give consent for themselves.
“Children and adolescents falling under the aforementioned age group, and their families, are encouraged to visit vaccination sites across the country to get vaccinated,” he concluded.
Meanwhile, the country’s health ministry in a daily COVID-19 update on Monday said, with the introduction of the Sinopharm 3rd dose, so far only 33 people have received the additional primary dose.